On a Southern California spring morning in 1973, a tanker truck driver jackknifed his rig and dumped the agricultural fumigant he was transporting onto a city street. A Los Angeles Fire Department emergency response team spent four hours cleaning up the chemical, 1,3-dichloropropene, or 1,3-D, a fumigant sold as Telone that farmers use to kill nematodes and other soil-dwelling organisms before planting.
Seven years after the spill, two emergency responders developed the same rare, aggressive blood cancer—histiocytic lymphoma—and died within two months of each other. In 1975, a farmer who’d accidentally exposed himself to 1,3-D repeatedly through a broken hose was diagnosed with another blood cancer, leukemia, and died the next year.
Within a decade of the men’s deaths, described as case studies in JAMA Internal Medicine, the National Toxicology Program, or NTP, reported “clear evidence” that 1,3-D causes cancer in both rats and mice. The finding led the U.S. Environmental Protection Agency to classify the chemical as “likely to be carcinogenic to humans” the same year, 1985. So it wasn’t a surprise when researchers at the University of California, Los Angeles reported in 2003 that Californians who’d lived at least two decades in areas with the highest applications of 1,3-D faced a heightened risk of dying from pancreatic cancer.
Yet EPA’s Office of Pesticide Programs’ Cancer Assessment Review Committee, or CARC, concluded in 2019 that 1,3-D—originally embraced by tobacco companies for its unparalleled ability to kill anything in soil that might harm their plants—isn’t likely to cause cancer after all.
In doing so, EPA, whose mission is to protect human health and the environment, rejected the human evidence, calling the UCLA study “low quality.” It also dismissed the authoritative NTP study and studies in lab animals that documented 1,3-D’s ability to damage DNA, a quintessential hallmark of cancer.
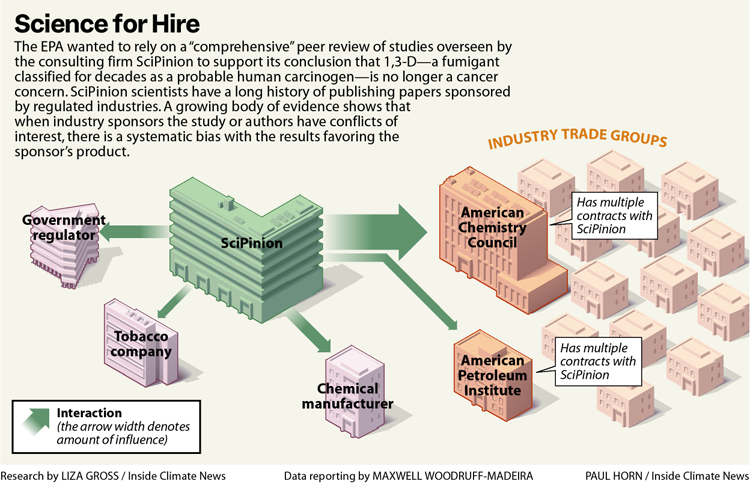
Instead, EPA’s CARC relied on studies provided by Dow AgroSciences (now called Corteva), the primary manufacturer of 1,3-D, and proposed a review of evidence linking the fumigant to cancer by SciPinion, a consulting firm hired by Dow, as an external peer review of its work. The decision to entrust external review to a Dow contractor has drawn repeated criticism, including from the agency’s watchdog, the Office of Inspector General, or OIG.
“During EPA’s search of the open literature, a comprehensive third-party peer review of the cancer weight-of-evidence assessment that considered toxicokinetics, genotoxicity and carcinogenicity data for 1,3-D was conducted and published in 2020 by SciPinion,” said agency spokesperson Timothy Carroll. EPA argued that the SciPinion review satisfied the criteria for an external review, Carroll said, and that another panel would have arrived at the same conclusion, given the specialized expertise required.
The OIG had recommended EPA conduct an external peer review of its 1,3-D cancer risk assessment in a 2022 report that outlined several problems with the agency’s process. An external review, the OIG said, requires “independence from the regulated business,” again noting the deficiency in a new report released in early August.
The scientists who run SciPinion have long consulted for manufacturers of harmful products, often publishing studies that deploy computer models to question the need for more protective health standards.
Inside Climate News reviewed 159 scientific articles published by one or both of the principal SciPinion scientists, Sean Hays and Christopher Kirman. Papers often re-evaluated evidence of health risks or advocated using alternative methods to “refine” risk estimates for dozens of toxic substances, most worth billions of dollars in sales a year, including toxic metals (like chromium, used to make stainless steel), solvents (like benzene), pesticides, flame retardants and PFAS, also known as “forever chemicals.” Of the 130 papers with a funder listed, 82 percent were sponsored by corporate interests, either the producer of the substance under study or the producer’s trade group, including the American Chemistry Council, the Chlorine Chemistry Council and the American Petroleum Institute.
Industry players are hiring SciPinion to do risk assessments on their own products, said Tess Legg, an expert on the risks of corporate influence on science with the Tobacco Control Research Group at the University of Bath in England. “They’re what some would call a ‘product defense team.’”
Papers published by SciPinion scientists, who also provide expert witness testimony and “product stewardship” services through the consulting firm Summit Toxicology, rarely conclude that toxic chemicals, including known carcinogens, need stricter regulations. Instead, they often argue for safety thresholds that allow higher levels of exposure, repeating an argument made by fossil fuel and petrochemical companies since U.S. regulatory agencies were established in the 1970s.

“They are all industry consultants and will tell you, ‘Oh, no, we just follow the science,’” said Linda Birnbaum, a world-renowned toxicologist who spent nearly 20 years at EPA and until 2019 directed two of the government’s leading environmental health research agencies, the National Institute of Environmental Health Sciences and the National Toxicology Program. “But if they don’t say things that make industry happy, guess what? They’re not going to get hired to do the next job,” said Birnbaum, now a scholar in residence at Duke University’s Nicholas School of the Environment.
Ultimately, Birnbaum said, “we need unconflicted external peer review for regulatory decision-making.”
Several authoritative science research bodies have repeatedly classified 1,3-D as a likely human carcinogen, including the National Toxicology Program and the International Agency for Research on Cancer, or IARC.
Growers applied more than 60 million pounds of 1,3-D to fields in 2018, according to the most recent data. In downgrading 1,3-D’s cancer status, EPA’s CARC sanctioned a 90-fold increase in the exposure level previously considered “an unreasonable risk.”
The decision will expose agricultural communities and farmworkers, who already live and work alongside the highest applications of 1,3-D and other dangerous pesticides, to even higher levels of a fumigant independent researchers have shown damages DNA in cells and lab animals, and linked to cancer and respiratory problems, including asthma, in humans. Even tiny, temporary spikes in 1,3-D concentrations increase the need for emergency asthma treatment, researchers have found.
SciPinion’s Hays and Kirman did not agree to an interview or respond to specific questions about Inside Climate News’ findings. “You seem unwilling to report the facts about how we produce trusted, independent science that upholds the highest standards of scientific integrity,” Hays said in a statement. “So here’s what we hope you’ll include for any readers of your story: We created SciPinion to be a voice for the scientific community, to provide a path for scientists to give their honest and uninfluenced opinions on the tough science matters and to renew trust in science. We fight back against biased and unfounded attacks on science experts. When experts fear retribution and attack by reporters and activists, they refuse to speak up on science issues. Attacks on scientists for their opinions is a disservice to science and civil discourse.”
When Inside Climate News first contacted Hays and Kirman, the firm’s growth executive, Tyler Carneal, replied, saying SciPinion strives to be a resource for journalists. He then pointed to EPA’s response to the 2022 OIG report as “the best resource.”
The OIG not only recommended EPA secure external peer review of the 1,3-D risk assessment but also charged the agency with failing to adhere to standard operating procedures and relying on scientific analysis techniques that lacked clear guidance. Such deficiencies, the OIG concluded in the report, “undermined scientific credibility and public confidence.” The OIG made nine recommendations for the agency to restore its credibility.
“EPA remains committed and continues its mission to protect human health and the environment, while maintaining scientific integrity, transparency to all stakeholders and decisions grounded in sound, high-quality science,” Michal Ilana Freedhoff, assistant administrator for the Office of Chemical Safety and Pollution Prevention, wrote in response to the OIG report. She said the agency will be taking action “to further strengthen our continued efforts in these areas.”
Over the past two years, EPA proposed steps to comply with the OIG’s recommendations. EPA’s Carroll maintains that the agency is no longer relying on SciPinion’s review. Yet the new OIG report released August 7, flagged one recommendation as unresolved: the need for an independent external peer review of the 1,3-D cancer assessment.
Downplaying Risks
Carcinogens are traditionally regulated using a “no threshold” approach, which assumes there is no safe level of exposure. Substances that cause other health problems were assumed to have a level below which they do not cause harm, but scientists now realize even non-carcinogens may carry risks at low doses.
EPA’s re-classification “dangerously ignores science and downplays the risks individuals face when they are exposed to 1,3-D,” wrote the attorneys general of seven states and the District of Columbia in a 2020 letter to the Office of Pesticide Programs. Top law enforcement officers from New York to California charged agency officials with omitting studies linking 1,3-D to cancer in humans, and ultimately relying “entirely on industry-sponsored studies” and “crediting an unsupported Dow theory” to ignore cancer responses at certain doses. Dow did not respond to repeated requests for comment.
The decision also triggered multiple complaints to the Office of Inspector General, which highlighted the attorneys’ general letter in its report.
In one complaint, Timothy Whitehouse, the executive director of Public Employees for Environmental Responsibility, or PEER, said misrepresentations and omissions by EPA officials “put applicators of the fumigant and the public at grave risk, given that 1,3-D is one of the nation’s most-used pesticides.”
Another complaint, submitted to the OIG hotline and obtained through a public records request, warned that chemical manufacturers are pressuring EPA to state that carcinogens have thresholds below which the chemicals are safe, “which is not protective of human health.”
PEER, which represents whistleblowers at public agencies, detailed several scientific flaws with EPA’s review in its complaint, including a search of the biomedical literature that didn’t use the chemical’s full name, 1,3-dichloropropene, and turned up just eight studies.
“EPA has recognized that there was an oversight in the original literature search for the CARC review, such that while the search terms used were correct, some synonyms for the chemical name were not included,” said EPA spokesperson Carroll.
Yet a document obtained through a public records request—and EPA’s 2019 draft human health risk assessment—shows that the original search terms did not include the chemical’s full name, which several scientists told Inside Climate News is “just a bad search.”
“These are not honest mistakes and carry the earmarks of deliberate malfeasance,” Whitehouse said in a press release about the complaint.
EPA failed to follow its own procedures for searching the scientific literature, the OIG report concluded. The watchdog suggested EPA conduct a new search to restore its scientific credibility.
A new search with the chemical’s full name returned 103 studies. But EPA concluded that none of the additional studies impacted 1,3-D’s cancer classification.
Yet independent scientists told Inside Climate News the expanded search included peer-reviewed papers that provide evidence 1,3-D should be assessed as a “no threshold” carcinogen because it harms DNA. In one study, 1,3-D damaged DNA in liver cells. In another, it “induced DNA damage in all of the organs studied.”
EPA gave these studies lower scores in the weight of evidence review because they revealed only initial damage to DNA, which may have initiated processes like DNA repair that minimize the harm, said Carroll on behalf of EPA’s pesticide office. To avoid underestimating risks, he said, EPA applied an uncertainty factor, or safety margin, of 100, which accounts for differences between rodents and people and within the population.
Experts in health and risk assessment have been arguing against this type of weight of evidence analysis for years because they don’t allow decision-makers to adopt a health protective approach, said Adam Finkel, clinical professor of environmental health sciences at the University of Michigan School of Public Health and former director of Health Standards Programs at the U.S. Occupational Health and Safety Administration.
If you synthesize all of the evidence in a way that removes uncertainty around the findings, Finkel said, you’re not giving regulators who want to take a health protective approach the information they need.
The National Academies told EPA in 2014 that “weight of evidence” is far too vague to have any scientific value, said Nicholas Chartres, an expert on reducing bias in risk assessments at the University of Sydney. “When you see ‘weight of evidence,’” he said, “it’s a red flag.”
CARC also dismissed the 1985 NTP study that formed the basis of the agency’s original decision to classify 1,3-D as a probable human carcinogen, accepting Dow’s argument that because the old Telone formulation included a stabilizer that mutates DNA, it was impossible to assess 1,3-D’s cancer effects in the rats and mice. Yet EPA had previously determined that even if the stabilizer had caused some of the tumors, its contribution would have been small.
SciPinion and Corteva (formerly Dow) scientists repeated the argument to dismiss studies with the stabilizer in separate weight of evidence reviews minimizing 1,3-D’s cancer risk published on the same day in 2021.
Finkel, one of the scientists on the SciPinion 1,3-D panel who does not consult for industry, said it was wrong to discount the NTP studies. It is simply improper to ignore 21 carcinomas of the bladder, which NTP called “clear evidence of carcinogenicity in female mice,” because of a trace amount of an impurity that has never been associated with any bladder carcinomas, Finkel believes.
The stabilizer is simply “too weak a carcinogen to cause the tumors,” Finkel told Inside Climate News. “It doesn’t add up.”
In 2021, two years after EPA’s CARC downgraded 1-3-D’s cancer status, the National Toxicology Program—the nation’s preeminent toxicology research arm—again said the chemical is “reasonably anticipated to be a human carcinogen.”
“We’re extremely concerned that EPA’s pesticide office is captured by industry, that its science is not trustworthy and that its staff are supposed to deliver for the industry, as opposed to protecting the public health,” Whitehouse, a former EPA enforcement attorney, told Inside Climate News.
“EPA rejects the inappropriate and unfounded notion that the agency’s career staff and pesticide registration decisions are influenced by external interests,” said Carroll. “Additionally, the underlying assertion suggesting that any errors by career staff are deliberate is a ridiculous and unfounded attack on scientists who have in most cases spent their entire careers as civil servants working to protect people.”
“We’re extremely concerned that EPA’s pesticide office is captured by industry, that its science is not trustworthy and that its staff are supposed to deliver for the industry, as opposed to protecting the public health.”
The agency has updated its procedures to avoid making errors in literature searches and has addressed other OIG recommendations to increase transparency in documentation and decision-making, he said.
Independent scientists also told Inside Climate News about other serious flaws in EPA’s assessment of 1,3-D.
Beate Ritz, an expert on occupational epidemiology and pesticide exposure at UCLA, led the California study linking 1,3-D to pancreatic cancer deaths that EPA dismissed as “low quality.” EPA rejected her study partly by arguing that living near pesticide applications doesn’t mean a person is exposed.
“But we know it does mean exposure,” Ritz said. “We have enough studies where we have done dust sampling or EPA itself has.”
Scientists working with pesticide regulators under California’s EPA office placed monitors on three elementary schools in a small Central Valley farming town. They collected hundreds of samples over the course of more than a year and found unsafe levels of several pesticides, including 1,3-D. When they compared their results with pesticide application records, Ritz said, “the overlap was amazing.”

What was sprayed on farms around the community could be measured on top of elementary schools, which means it got into households, too, Ritz said. Other researchers sampled dust in homes and urine in residents and showed that application records could predict what they found in dust and urine, she said. “So definitely, this stuff gets into the homes, it gets into dust, it gets into people and it comes out of people.”
Yet EPA is increasingly disregarding epidemiology data, Ritz said. With large farming populations chronically exposed at high levels to multiple agents, she said, clearly frustrated, “that’s a bad road to go down.”
Air pollution studies show that what monitors detect can be assumed to be reaching the population, Ritz said. “And we have been regulating air pollution forever based on these studies. Why not pesticides?”
EPA classifies a substance as “likely to be carcinogenic to humans” based on multiple criteria, including evidence of cancer effects in animal experiments in “more than one species, sex, strain, site or exposure route, with or without evidence of carcinogenicity in humans.”
EPA acknowledged that 1,3-D caused liver tumors in rats but dismissed the mouse lung tumors by accepting Dow’s theory that if tumors occur above a dose determined by a computer model, they’re not relevant to humans. SciPinion’s Hays co-authored a paper with Dow scientists promoting the approach for agrochemical risk assessment in 2016. That approach is not accepted by the broader scientific community, and independent researchers said there is no scientific basis for EPA to rely on it to discount evidence that 1,3-D causes lung tumors in mice.
They basically said the lung tumors don’t count as a second site of cancer because they happened above this inflection point in the exposure, said John Bucher, a leading toxicologist who recently retired from the National Toxicology Program after nearly four decades. They’re using that conclusion to downgrade the cancer status, but they’re just ignoring the fact that there’s a cancer response in the second animal, Bucher said.
“That’s a violation of scientific principles,” he said. “You can’t take away the fact that there was a cancer response just because it occurred at a dose that was not in the linear range. That’s just ridiculous.”
The OIG recommended that EPA conduct an external peer review—that is, by independent scientists without ties to the chemical’s manufacturer—of its cancer risk assessment to restore confidence in its scientific credibility.
EPA’s Carroll said the agency is working to “arrive at a mutually acceptable resolution” to the OIG’s request for an external peer review, is committed to transparency and scientific integrity and will continue to use sound science in the decision-making process.
But so far, EPA has not proposed a solution for an external review that the OIG has agreed to.
Conflict-Ridden Science
On its website, SciPinion tells prospective clients it will support their scientific claims, “quash unfounded criticisms” and manage the threat of regulatory action, litigation or public dissent that threatens a product’s acceptance. “SciPinion builds trust in the scientific process,” its website says, through a trademarked, certified peer-review process with a panel of global experts that will “validate your science.”
Among the examples of its ability to quickly assemble expert panels to serve clients’ needs, SciPinion includes the 1,3-D cancer reclassification along with two papers, funded by the American Chemistry Council, on PFAS, ubiquitous, highly persistent and toxic “forever chemicals.” One of the papers, a 2022 peer-reviewed weight of evidence review, discounts the immune toxicity of PFAS, rejecting evidence from several studies of children. A second peer-reviewed report, published last year, argues against regulating PFAS as a class. The American Chemistry Council promoted the study on its web site without mentioning it sponsored the research.
“One aspect of SciPinion that many find intriguing is our robust process and our triple-blinded approach to assure untainted expertise, delivering purity in scientific opinion,” said SciPinion’s Carneal, speaking on behalf of Hays and Kirman in a statement to Inside Climate News. “In part, this is why we are so widely trusted—obtaining objective scientific opinion is our core mission.”
EPA’s Carroll said agency staff decided SciPinion’s review process was similar enough to that of its scientific advisory panel, which provides independent advice to the pesticide office, to count as an external reviewer.
Yet their conclusions often clash with those of independent researchers.
More than 200 environmental and health scientists urged agencies to restrict all but essential uses of PFAS as a whole in 2015, citing evidence of numerous health effects, including tumors in multiple systems, liver toxicity, neurobehavioral problems and disruption of immune and endocrine systems among others. Many of the same scientists later called on governments and industry to treat the thousands of PFAS chemicals as a class to protect public health and the environment from toxic chemicals that never break down.
Philippe Grandjean, a Danish professor of environmental medicine, led the studies of PFAS and immunotoxicity that the SciPinion panel rejected. Grandjean first reported in a 2012 study of hundreds of children in Denmark’s Faroe Islands that PFAS exposure reduced the immune response to tetanus and diphtheria vaccines below the level needed for long-term protection. He has had similarly troubling results in follow-up studies.
Grandjean said he doesn’t take the SciPinion conclusion seriously, “given that an authority like IARC has suggested that immunotoxicity could be the mechanism for PFAS carcinogenicity,” referring to evidence that the chemicals impair the body’s ability to fight cancer.
Such examples show why having expert panels with conflicts of interest is so problematic, said Chartres of the University of Sydney. Chartres recently reviewed another SciPinion expert panel evaluation of a solvent that was sponsored by a trade group.
“On the surface, it looks like they’re evaluating the science, it’s scientifically defensible and it’s rigorous,” Chartres said. But on closer inspection, it’s clear that they changed the way legitimate review tools work to rate and score studies, he said. “And so basically, they can throw out and cherry pick studies however they want to.”
Chartres’ assessment of SciPinion’s approach reflects what Finkel, the occupational and environmental health expert, experienced as part of the firm’s 1,3-D panel. Finkel thinks his input as part of a five-member subpanel evaluating the weight of evidence was marginalized in SciPinion’s published paper.
He feels the process did a good job of airing the controversies but also manipulated what reviewers saw and, more importantly, what they said. “To summarize the whole thing as saying everybody pretty much agreed is disingenuous,” Finkel said. “Not everybody did agree.”
For example, on the questions of whether 1,3-D should be assessed using a threshold model, three reviewers on his panel said yes but two said no, with a “fairly strongly disagree” response, he said. “That’s not consensus at all.”
Finkel was also puzzled by the lack of an option that NTP and IARC uses in their evaluations: possibly (or probably) carcinogenic to humans. “I would have chosen this option if available,” he told SciPinion.
Finkel appreciated the ability to engage in exhaustive discussions about the process, but added, “I left with a bad taste, because I don’t think they were honest about reporting the disagreements.”
In 2000, an EPA science advisory board considered analyses done by SciPinion scientists (then with other consulting firms) in reviewing the agency’s risk assessment for dioxin, a known human carcinogen. The SciPinion scientists’ analysis argued that dioxin, considered one of the most toxic synthetic substances ever identified, had a cancer risk threshold an order of magnitude above what most people are exposed to. But when academic scientists with no ties to the manufacturer reexamined the analysis, they found a flaw that, once corrected, revealed no safe threshold.
“We know empirically that when industry sponsors the study, or when you have authors with a conflict of interest, you get a systematic bias with the results being in favor of the sponsor’s product.”
Dow nevertheless relied on one of the SciPinion scientists to argue that dioxin, a by-product of pesticide manufacturing, should be regulated using a threshold approach in 2008.
Regardless of what evidence has been evaluated, Chartres said, “we know empirically that when industry sponsors the study, or when you have authors with a conflict of interest, you get a systematic bias with the results being in favor of the sponsor’s product.”
SciPinion’s Hays did not address how papers published by independent scientists and those by SciPinion scientists arrived at opposite conclusions, but said in a statement that the Inside Climate News email “seems to ask us to respond to false accusations from unnamed sources. It reads as a biased character attack and exactly the kind of engagement we created SciPinion to protect science experts from receiving.”
Marshaling Evidence of a Threshold
U.S. regulators have historically classified 1,3-D as likely to be carcinogenic to humans based on rodent studies conducted in the 1980s, SciPinion scientists wrote in their weight of the evidence assessment, referring to the NTP study. Contemporary studies, they explained, led them to conclude that 1,3-dichloropropene “is not mutagenic and not carcinogenic below certain doses, pointing to a threshold-based approach for cancer risk assessment.”
In the paper’s acknowledgements, SciPinion thanked the 14 “independent experts” for reviewing their analysis while noting that Dow AgroSciences had an opportunity to review the manuscript “to ensure clarity and completeness.” Hays said SciPinion used an “outside auditor who is a former FDA scientist and served in the Obama White House to help select the panel of experts.”
That SciPinion did a weight of evidence review of the cancer literature using a so-called expert panel rings alarm bells, said Legg of the University of Bath’s Tobacco Control Research Group. The expert panel was chosen by the authors of the review who were funded by the manufacturer of the product. “So far, very conflicted,” she said.
They managed to get 14 experts, but it’s possible that many independent experts would have bowed out once they learned the review was funded by the product’s manufacturer, Legg said. Two-thirds of the panel SciPinion assembled have ties to regulated industries, including Dow and two other agrochemical giants, Monsanto and Syngenta, a review of their affiliations shows.
“The cancer classification subpanel was entirely comprised of former U.S. government scientists,” Hays told Inside Climate News.
Three of the five sub-panelists are former EPA scientists who consulted for the American Chemistry Council or pesticide companies after leaving the agency. “What we know from our science-for-profit model and from other evidence,” Legg said, “is that the best way to create reliable and trustworthy evidence on product harms is not to use science that is funded by the manufacturers of those products.”
In their 2021 paper “The Science for Profit Model—How and why corporations influence science and the use of science in policy and practice,” published in the journal PLOS ONE, Legg and her colleagues analyzed corporate attempts to influence science and policy across eight sectors, including the fossil fuel, tobacco and chemicals and manufacturing industries.
“What we found was that industries were funding science not to create science in the public interest,” she said, “but to maximize profits.”
Finkel said his initial SciPinion contract did not mention who sponsored the review. But he’s more troubled that Dow had the right to review the manuscript. “‘Clean’ contracts with a manufacturer or other interested firm should not allow advance notice or rights of review,” Finkel said.
“What we found was that industries were funding science not to create science in the public interest, but to maximize profits.”
The SciPinion expert panel process echoes what happened when Public Health England relied on the opinions of a small “independent” expert panel to announce that e-cigarettes were 95 percent safer than combustible cigarettes, Legg said. The Lancet, a highly regarded medical journal, criticized the report’s “extraordinarily flimsy” evidence base and said conflicts of interest raised serious questions about the panel’s conclusion.
Just as the tobacco industry expanded the reach of its message through an echo chamber of industry-friendly groups and conservative think tanks, SciPinion’s evaluation of 1,3-D was promoted by a “pro-science” third-party, the American Council on Science and Health, which presented the cancer review sponsored by Dow as independent.
That’s “incredibly problematic,” Legg said, because the average person reading such a third-party post would have “absolutely no idea” the review was industry-funded.
SciPinion’s 1,3-D weight of evidence analysis cites a method for managing expert panels that “maximize expertise” and minimize bias.
That method, it turns out, is their own, presented in the 2019 paper, “Science peer review for the 21st century: Assessing scientific consensus for decision-making while managing conflict of interests, reviewer and process bias.” In the new model, developed for cigarette manufacturer Philip Morris International, SciPinion demonstrated how to “reduce sources of bias” in evaluating the company’s “modified risk” tobacco product. Philip Morris used the peer reviews as independent validation of the science underlying its claims of reduced harm submitted to regulators at the Food and Drug Administration.
“We make our science publicly available and encourage any and all to scrutinize our work and arrive at their own conclusions, just as the FDA has done with respect to our modified risk tobacco product applications,” said Corey Henry, a Philip Morris International spokesperson.

That Philip Morris is funding research on how to do such a critical part of the scientific process as peer review—and enlisted SciPinion to do it for them—“should ring alarm bells,” said Legg. The tobacco industry has been working to manipulate science in its favor for decades, she said, for example, by trying to define what constitutes “sound” or “junk” science, ostensibly to boost scientific integrity when in reality it was trying to prevent regulations on secondhand smoke.
Since then, researchers have documented the disproportionate power of corporations to influence policy across sectors, as Legg showed in the science-for-profit model. “Not much has changed,” she said.
A Manufactured Controversy
The concept that carcinogens have no safe exposure level is based on evidence going back decades that susceptibility varies due to genetic, physiological and environmental factors and timing of exposure (for example, pregnancy, prenatal development and early childhood). Environmental health scientists have repeatedly shown that while it’s possible to design a model to show there’s a threshold below which a chemical exposure could be deemed safe, doing so may greatly underestimate risk if the computer model does not reflect real-world conditions.
The whole idea of looking for thresholds, or dividing the world into thresholds and non-thresholds, is a stupid idea, said Finkel. What matters, he said, is where an exposure becomes dangerous.
“There was this movement, in part promoted by the chemical industry, that we should be treating everything like we did with noncancer, that there’s a threshold value,” said Tracey Woodruff, a former EPA senior scientist who now directs the Program on Reproductive Health and the Environment at the University of California, San Francisco.
This story is funded by readers like you.
Our nonprofit newsroom provides award-winning climate coverage free of charge and advertising. We rely on donations from readers like you to keep going. Please donate now to support our work.
Donate NowThe only things they agreed could be treated as having no safe dose were cancer-promoting chemicals that damage DNA, otherwise everything had to have a threshold, Woodruff said. “And that has just simply not been proven.”
EPA’s Cancer Assessment Review Committee re-classified 1,3-D to “suggestive evidence of carcinogenic potential” based on a weight of evidence evaluation of all available studies, which included studies identified in the open literature and updated data submitted by Dow, said agency spokesperson Carroll. The committee concluded that it didn’t need to estimate human cancer risks because a safety level set for all chronic health effects would also protect against cancer, he said.
But mounting evidence shows that even chemicals that don’t cause cancer may have no safe level of exposure in sensitive groups. That’s why the National Academy of Sciences, or NAS, an authoritative body of top scientists, recommended in a 2009 report that EPA should evaluate both noncancers and cancers using a no-threshold model, unless there’s clear evidence of a threshold above which a substance becomes hazardous, Woodruff said.
Finkel, who contributed to the NAS report, said it reflected the risk assessment field’s movement away from the paradigm of finding a number and dividing it by another number to say an exposure is safe. “They used to call that ‘divide by 100 and pray,’ because we had no idea whether the original level was safe to begin with, and whether the factor of 100 was enough to make it safe enough,” Finkel said.
But SciPinion panels keep pushing the exact opposite, he said. “They want to make cancer risk assessment look more like the simplistic old non-cancer risk assessment paradigm that was developed in the ’50s.”
Dow first lobbied EPA to reclassify 1,3-D using a threshold in 1998. That same year, in a newsletter read by policymakers, SciPinion scientists promoted PBPK modeling as a way to “improve the regulation of exposure to toxic compounds” and reduce uncertainty in estimating risks of exposure to carcinogens.
Then, in 2013, Dow formally asked EPA to reconsider 1,3-D’s cancer status using a threshold approach, arguing that a review by an independent expert concluded that the chemical does not directly damage DNA. Dow did not mention that its independent expert was a litigation consultant for Philip Morris who argued in a class-action suit against the tobacco giant that Marlboro lights reduced disease risk, contrary to the findings of a leading medical research body.
Dow did not respond to requests for comment.
More than 30 years ago, Dow, as part of a coalition of chemical and fossil fuel companies, sought to “improve” EPA’s risk assessment of another dangerous product, benzene—first linked to leukemia in people in the 1920s—by “refining the data and assumptions,” which showed up to 10 times less risk.
Benzene, a component of crude oil used to make motor fuels, pesticides and other products, was classified as a carcinogen by IARC in 1979.
The decision followed clear evidence benzene caused cancer in lab animals and a 1977 occupational health study establishing a link between benzene exposure and leukemia in people. The study, led by Peter Infante, an epidemiologist who worked for decades at federal research and regulatory agencies, including 24 years at the Occupational Safety and Health Administration, or OSHA, showed that workers at an Ohio Goodyear plant exposed to benzene while making a rubberized food wrapping called Pliofilm had up to ten times higher risk of developing leukemia than the general population. OSHA issued an emergency standard to reduce workplace benzene standards from 10 parts per million, or ppm, to 1 ppm.
The American Petroleum Institute, or API, quickly challenged the lower standard in court and attacked regulators’ assumptions of no safe exposure levels for carcinogens as “opinion.” The API convened a task force that included a Dow executive and hired consultants to draft reports showing why thresholds are the most appropriate way to estimate risks from exposure to benzene, documents released through litigation show. A task force action item calls for finding a consultant to run a PBPK model to “rebut the studies showing genotoxic effects in mice in the low ppm range.”

“There are all kinds of studies showing all kinds of chromosomal breakage with benzene,” said Infante. “It’s a genotoxic carcinogen.”
It’s been clear for years that any level of carcinogens can potentially harm vulnerable people, Infante said. Yet, he added, “the literature keeps getting polluted with nonsense.”
SciPinion scientists also published studies, sponsored by the API and the American Chemistry Council, that minimize the risks of benzene exposure. One reviewed evidence of potential associations between childhood leukemia and benzene, and found no evidence that environmental levels pose an increased risk. A second found that children are not likely to be at elevated risk of a rare form of leukemia, called acute myelogenous leukemia, or AML, from benzene.
But studies by independent scientists show that children who live next to gasoline stations, which emit benzene, have an elevated risk of leukemia, Infante said. “And benzene causes AML in adults. Why the hell wouldn’t it cause it in children? Children are usually more susceptible. That makes no sense.”
Again, Hays did not address the disconnect between the work of independent researchers and his firm’s. “What we most hope your readers appreciate is that SciPinion exists for the scientific community and to see that we will not stand for activist-reporters attacking scientists’ credibility rather than informing science-based discourse,” he said in a statement.
More than 50 years ago, the head of the National Institute of Environmental Health Sciences warned that even if an individual chemical has a threshold, it’s irrelevant when the world is increasingly awash in carcinogens that can interact to cause more harm than any one would alone.
Yet EPA stands by its decision that a chemical it considered a likely carcinogen for decades is not a cancer risk, while authoritative bodies at home and abroad continue to classify it as a probable human carcinogen. And though the agency insists it is no longer relying on SciPinion to justify its decision, it has yet to conduct a truly independent peer review.
The fact that a regulatory agency like the U.S. EPA leaned on a group that has so much industry money and support, said the University of Sydney’s Chartres, “well, that is egregious.”
About This Story
Perhaps you noticed: This story, like all the news we publish, is free to read. That’s because Inside Climate News is a 501c3 nonprofit organization. We do not charge a subscription fee, lock our news behind a paywall, or clutter our website with ads. We make our news on climate and the environment freely available to you and anyone who wants it.
That’s not all. We also share our news for free with scores of other media organizations around the country. Many of them can’t afford to do environmental journalism of their own. We’ve built bureaus from coast to coast to report local stories, collaborate with local newsrooms and co-publish articles so that this vital work is shared as widely as possible.
Two of us launched ICN in 2007. Six years later we earned a Pulitzer Prize for National Reporting, and now we run the oldest and largest dedicated climate newsroom in the nation. We tell the story in all its complexity. We hold polluters accountable. We expose environmental injustice. We debunk misinformation. We scrutinize solutions and inspire action.
Donations from readers like you fund every aspect of what we do. If you don’t already, will you support our ongoing work, our reporting on the biggest crisis facing our planet, and help us reach even more readers in more places?
Please take a moment to make a tax-deductible donation. Every one of them makes a difference.
Thank you,













